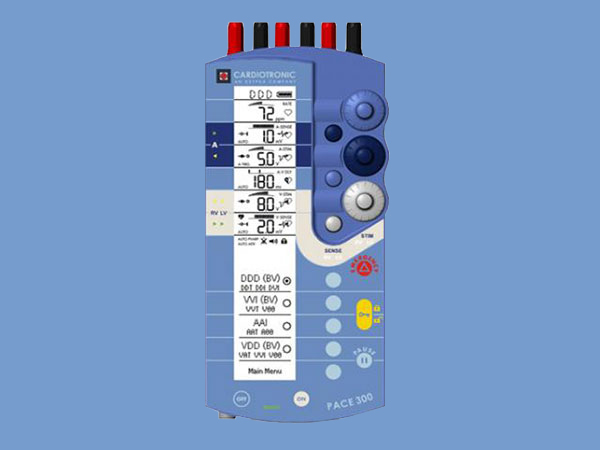
PACE 300
Three Chamber Temporary Cardiac Pacemaker
Product Description
The PACE 300™ is the world’s first three-chamber temporary cardiac pacemaker featuring biventricular (biV) pacing capability. The PACE 300 temporary cardiac pacemaker is designed to be used with cardiac pacing lead systems for temporary atrial, ventricular, A-V sequential, or biV pacing. The PACE 300 has applications where such pacing modes are indicated for therapeutic, prophylactic, and/or diagnostic purposes.
The PACE 300 is currently pending US market approval.
The novel technology and pacing options of the PACE 300 makes it the only choice for biV temporary pacing in the OR, ICU, and for electrophysiology studies:
- Three dedicated pacing terminals
- Fast access to three, dual, or single chamber pacing modes
- Auto Sense function automatically tracks P/R wave amplitudes and adjust atrial and ventricular sensitivities accordingly
- Statistical recording and analysis of pacer activity
- Interface with Pacemaker Clinic™ for automatic CRT optimization
Intuitive User Interface The user friendly interface of the PACE 300 allows for effortless patient management for atrio-ventricular synchronous pacing supplemented by two individually programmable ventricular pacing channels and a programmable inter-ventricular delay (VVD). The dedicated pacing terminals allow for untangled and easy identification of pacing leads into the heart, as well as having dedicated dial knobs to set individual amplitude and sensitivity settings for each chamber.
Statistical Results The PACE 300 has the capability to records and analyzes the activity of the pacer, including overall sensed cycles, paced cycles, and the percentage of paced cycles. Clinicians can use these statistical results to help determine the patient’s dependency on pacing.
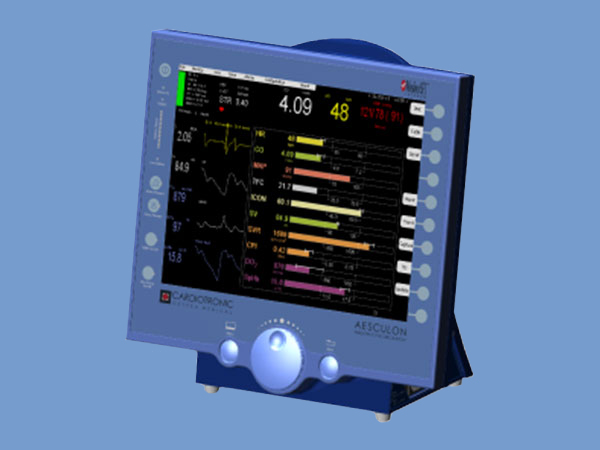
Pacer Optimization The PACE 300 can interface directly with Cardiotronic’s AESCULON® hemodynamic monitor (not included) using the Pacemaker Clinic™ application for fast and easy pacer optimization. With just one button push, Pacemaker Clinic automatically cycles through timing combinations and recommends optimal timing adjustments based on hemodynamic assessments for each combination. Data is displayed in a tabular format and can be printed as a report or exported to a PC.
Improvement of Using Cardiac Output and Biventricular Pacing Post-Cardiac Surgery Recent clinical publications have shown the added benefit in using temporary biventricular pacing, as opposed to standard dual chamber pacing in post-cardiac surgery patients.1,2,3 Furthermore, the optimization of temporary biV pacing has been shown to help treat patients with low output states.4,5
The increasing evidence pointing to benefits such as decreased morbidity and mortality rates in post cardiac surgery patients creates the perfect platform for the PACE 300.
Specific Ations:
Dimensions
212 mm x 96 mm x 51 mm(8.3” x 3.8” x 2.0”)
Weight
490 g (1.08 lb) including battery
Battery
Alkaline: 4 days (9.0V)
Basic Rate
30 – 220 ppm
High Pacing Rate
70 – 1000 ppm
Stimulation Amplitude
0.1 – 18 V
Pulse Width
0.05 – 1.5 ms
Sensitivity
Atrial: 0.2 – 20 mV & Ventricular: 1.0 – 20 mV
Refractory Period
Atrial: 250 ms & Ventricular: 250 ms
Pacing Modes
Primary: DDD (BV, R, L), VVI (BV, R, L), AAI, VDD (BV, R, L)
Secondary: D00 (BV, R, L), V00 (BV, R, L), A00, DVI (BV, R, L), DAI (BV, R, L), VAT (BV, R, L), AAT, DDD+AT (BV, R, L), DAT (BV, R, L), DDI (BV, R, L)